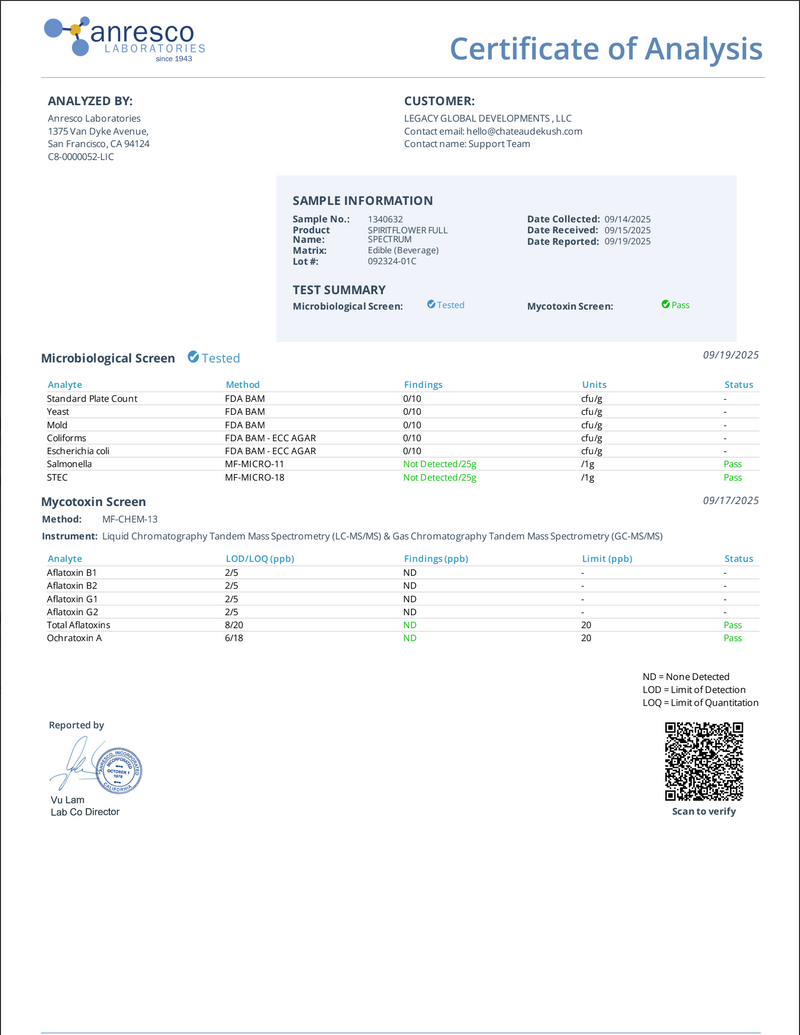
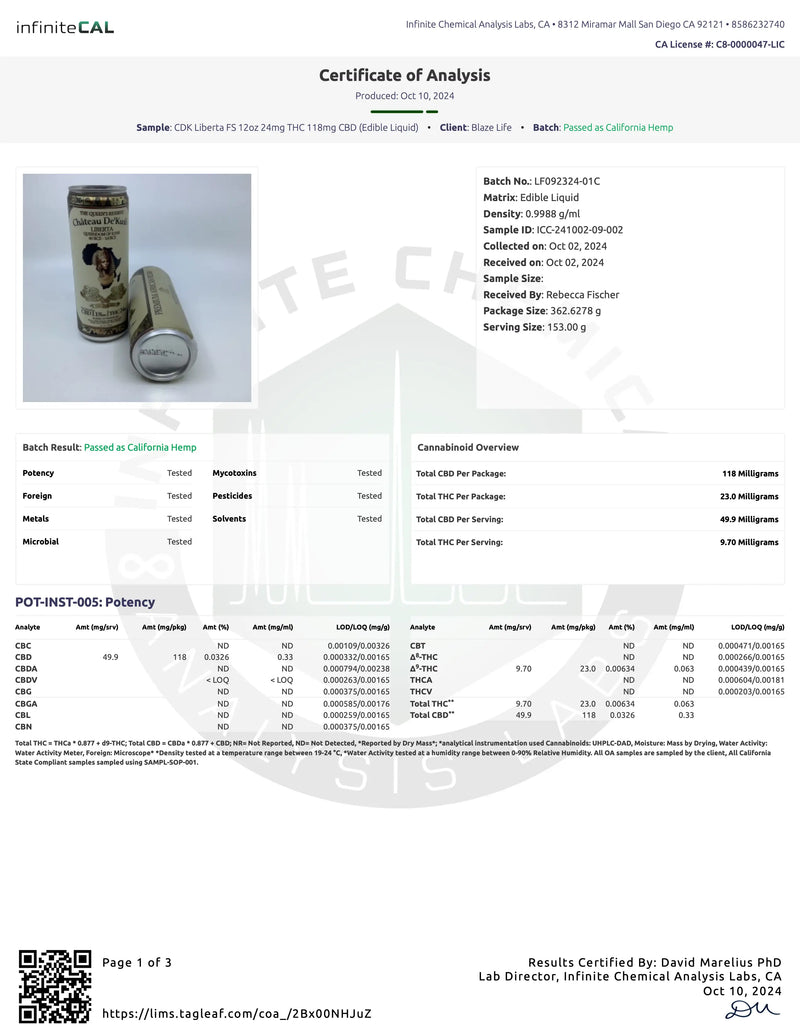
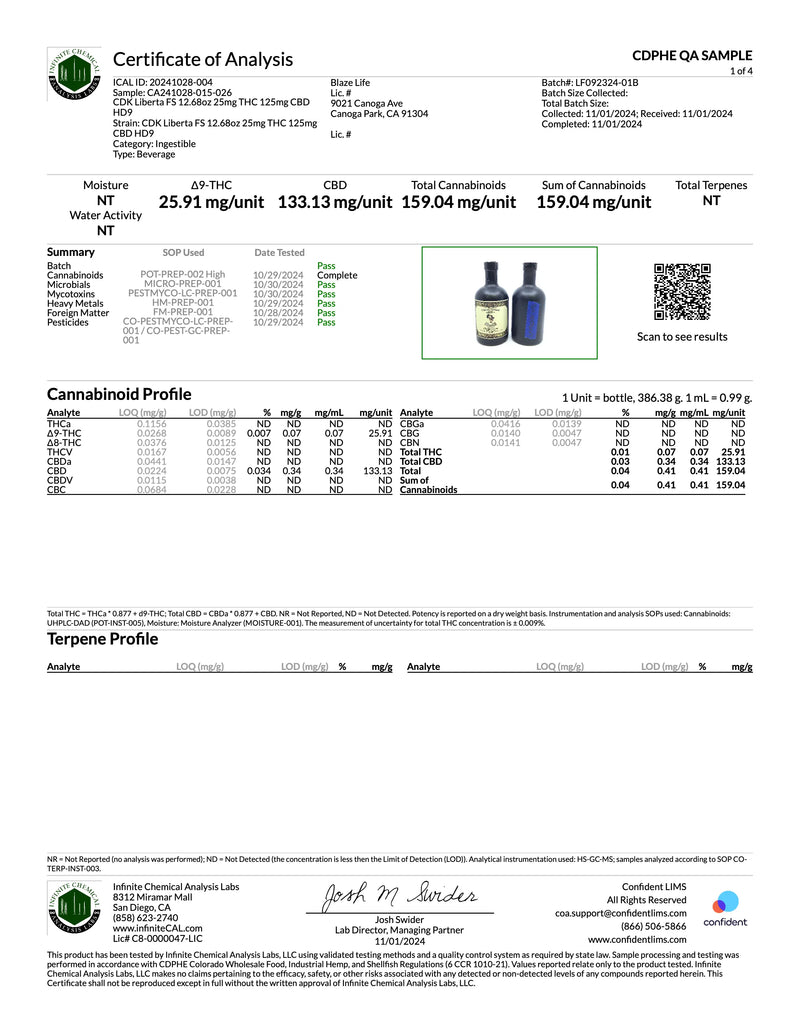
Certificates of Analysis
At Château De'Kush, quality and transparency are our top priorities. Our Certificates of Analysis (COA) provide detailed lab testing results for our products to ensure they meet the highest safety and quality standards.
What is a Certificate of Analysis?
A Certificate of Analysis is an official document issued by a third-party laboratory that verifies the contents, potency, purity, and safety of our products. Each COA includes comprehensive test results such as:
- Active ingredient levels
- Purity and contaminants
- Microbial testing
- Heavy metals screening
Why Are COAs Important?
COAs help guarantee that what’s listed on the product label matches what’s inside. They provide peace of mind to our customers by confirming:
- Compliance with industry regulations
- Absence of harmful substances
- Consistent product quality
- Accurate potency and ingredient profile